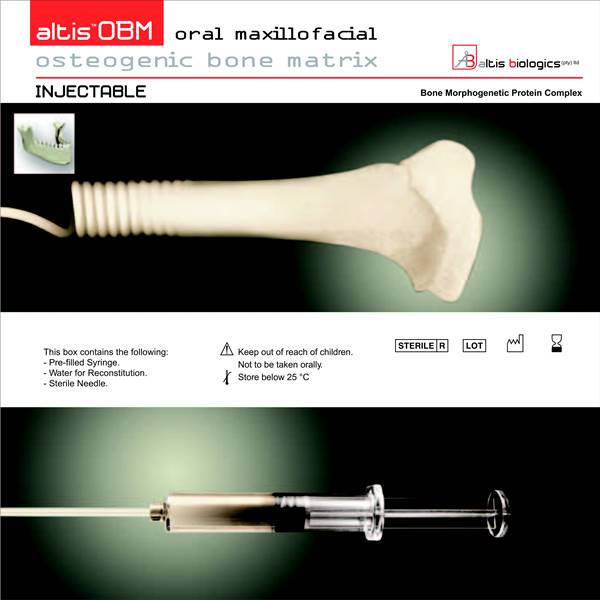
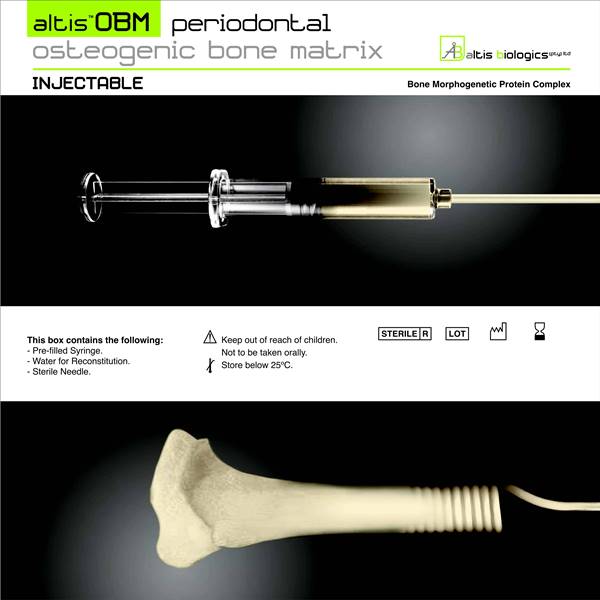
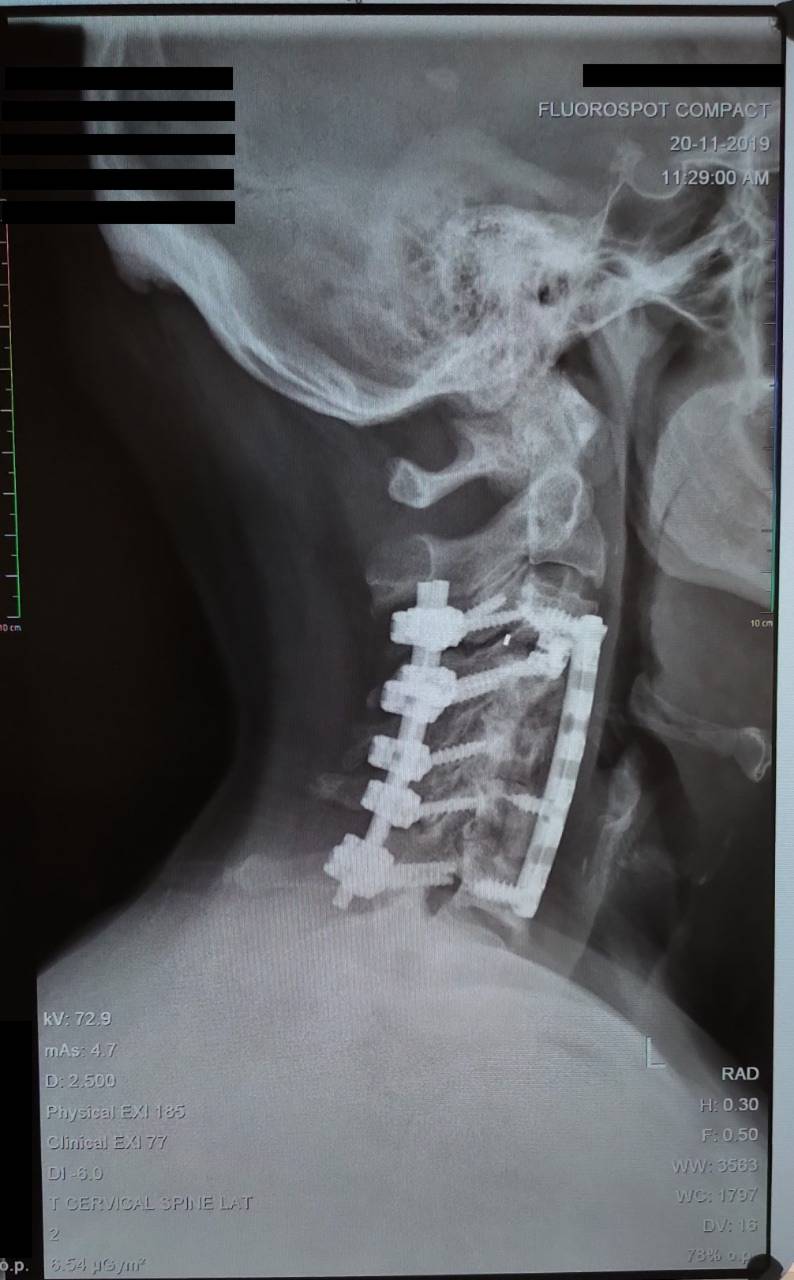
Altis Biologics
Technology Background
The technological basis underlying the Altis Osteogenic Matrix are the Bone Morphogenetic Proteins (BMP), a family of growth factors capable of inducing new bone formation commencing with days of implantation into human patients.
Play InfoPlay OCI-Altis
more info